Translation as a novel RNA silencing-based defense against foreign genetic elements
The Voinnet group (IMPB) describes, in "EMBO reports", a hitherto unknown translation-coupled mechanism whereby an evolutionary young Arabidopsis transposon is detected as a foreign entity owing to an unusually intense ribosome stalling event that funnels one of its mRNAs into RNA silencing.
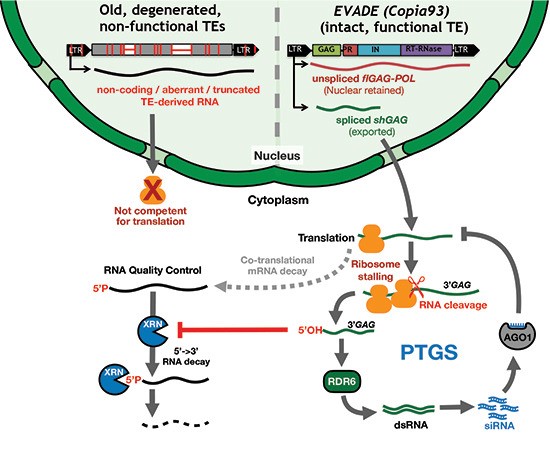
Plants use RNA silencing mediated by small RNAs (sRNA) to protect their genomes against transposable elements (TEs). This defense initially occurs post-transcriptionally via RNAi causing, in fine, DNA methylation and heterochromatinization on many Arabidopsis TE loci mostly consisting of degenerated relics of previous genome invasions. sRNAs spawned from this reservoir of inactive, methylated TE remnants likely provide identity-based immunity against sequence-related TEs. How, by contrast, de novo invading TEs (e.g. following horizontal transfer) with no such sequence-homology are primarily detected remains elusive.
Focusing on one of the few evolutionary young and autonomous TEs of Arabidopsis, called ÉVADÉ (EVD), Oberlin et al. (2021) now reveal that translation likely provides one such innate detection mechanism. They describe how an intense ribosome stalling event on one of EVD’s transcripts correlates with its cleavage at the precise initiation point of EVD-derived sRNA production. The unusual termini of the cleaved RNA (5’-OH and likely 3’-polyA-) prevent its degradation by RNA quality control, enabling, instead, its conversion into double-stranded RNA, the universal trigger of RNAi. The process, coined “Translation-dependent silencing” (TdS), illustrates how a lack of co-evolution between the host translation machinery and the open reading frames of invasive nucleic acids might ultimately unveil their “foreignness” and activate, in turn, genome-defensive RNAi.
Link to the paper in external page EMBO reports